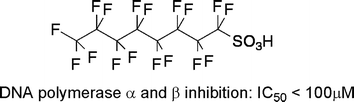
In this study, the chemical properties of organic acids as DNA polymerase inhibitors were examined. In total, we assayed the inhibitory activities of 23 compounds. We found that the DNA synthesis activity of DNA polymerase was usually reduced to less than 50% in the presence of 100 µM monoprotic acids, which have a Clog P value greater than 7.0 and a pKa value less than 5.4. With a minor modification these chemical properties applied to several organic fatty acids previously reported as DNA polymerase inhibitors. Moreover, we also examined the inhibitory activities of perfluorooctadecanoic acid (PFOdA) and perfluorooctanesulfonic acid (PFOS) against DNA polymerase β in detail. These compounds inhibited the polymerase activity of pol β competitively with template–primer DNA, and non-competitively with dNTPs. In addition, the 8 kDa domain-defective pol β was also sensitive to these compounds. Our results suggest that the inhibitory mode of action of PFOdA and PFOS is different from that mediated by the classic fatty acid inhibitors against DNA polymerase β.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...