On the mechanism of the Rh(ii)-catalysed cyclopropanation of alkenes†
Abstract
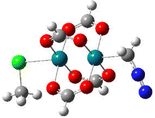
The mechanism of Rh(II)-catalysed cyclopropanation has been investigated computationally using Rh2(formate)4 as a model precatalyst, with the model organic substrates CH2N2 and C2H4 and MeCl as a model for coordinating

 Please wait while we load your content...
Please wait while we load your content...