Hydrogen-bonded donor–acceptor compounds for organic ferroelectric materials
Abstract
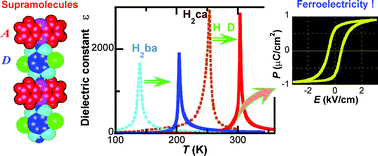
Organic ferroelectrics are multifunctional candidates for future organic electronic and optical devices. In spite of their potential, only a few organic compounds are known to exhibit a ferroelectric transition. The conventional approach to ferroelectrics, in general, relies on the use of asymmetric dipolar molecules and/or substituents. Recently, distinct design strategies have been developed using the molecular compounds of binary- or multi-components, combined with “non-covalent” forces: charge-transfer interactions and/or hydrogen bonding. This article focuses on the supramolecular systems of hydrogen-bonded acid and base molecules. Ferroelectricity and a significant dielectric response, as well as an antiferroelectric ordering induced by proton transfer, are demonstrated in the hydrogen-bonded chains composed of 2,5-dihydroxy-p-benzoquinone derivatives and nitrogen-containing aromatic bases.

 Please wait while we load your content...
Please wait while we load your content...