Concise enantioselective synthesis of abscisic acid and a new analogue†
Abstract
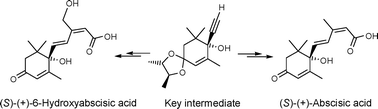
Short and high-yielding syntheses of enantiomerically pure (S)-(+) and (R)-(−)-abscisic acid are described. The syntheses proceed through key intermediates that preferentially recrystallise as single diastereoisomers for each enantiomer. This route allows the preparation of either enantiomer of abscisic acid in ca. 30% overall yield, and as demonstrated, gives access to an enantiomerically pure abscisic acid analogue.

 Please wait while we load your content...
Please wait while we load your content...