Asymmetric induction during photocyclization of chiral and achiral α-oxoamides within achiral zeolites†
Abstract
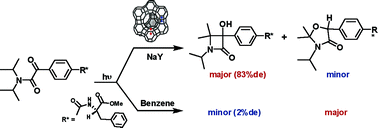
The photochemistry of 31 α-oxoamides capable of undergoing γ-hydrogen transfer has been examined within zeolites. These molecules, upon excitation, yield two products—a β-lactam and oxazolidinone—in solution, both resulting from γ-hydrogen transfer. While in benzene the major product is oxazolidinone, within an MY zeolite, the main product is a β-lactam. In this investigation, we have focused our attention on asymmetric induction in the formation of the β-lactam product. Two approaches—using a chiral inductor and chiral auxiliary—have been employed. While in solution, in the presence of chiral inductors, achiral α-oxoamides yield β-lactams with zero enantioselectivity; within zeolites, an ee of up to 44% has been achieved. α-Oxoamides appended with a chiral auxiliary gave β-lactams with less than 5% diastereoselectivity in solution while within zeolites, the same α-oxoamides gave the products with de’s of up to 83%. Such a remarkable influence of zeolites is attributed to an alkali ion interaction with the reactant α-oxoamides and to the confined environment of the zeolite interior. At this stage, we have not been able to provide a model with predictive power and further work is needed to understand this valuable asymmetric induction strategy.

 Please wait while we load your content...
Please wait while we load your content...