Traditional Morita–Baylis–Hillman reaction of aldehydes with methyl vinyl ketone co-catalyzed by triphenylphosphine and nitrophenol†
Abstract
In the Morita–Baylis–Hillman reaction of aldehydes with methyl vinyl ketone (MVK), we found that in the presence of a catalytic amount of phenol, the Lewis base triphenylphosphine can effectively promote the reaction to give the corresponding normal Morita–Baylis–Hillman adducts in good yields. The mechanism has been investigated by 31P NMR spectroscopy. The solvent and substituent effects were also examined.
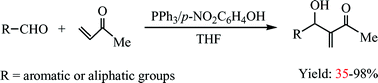

 Please wait while we load your content...
Please wait while we load your content...