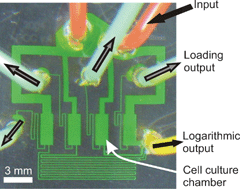
We present a microfluidic device for culturing adherent cells over a logarithmic range of flow rates. The device sets flow rates through four separate cell-culture chambers using syringe-driven flow and a network of fluidic resistances. The design is easy to fabricate with no on-chip valves and is scalable both in the number of culture chambers as well as in the range of applied flow rates. Using particle velocimetry, we have characterized the flow-rate range. We have also demonstrated an extension of the design that combines the logarithmic flow-rate functionality with a logarithmic concentration gradient across the array. Using fluorescence measurements we have verified that a logarithmic concentration gradient was established in the extended device. Compared with static cell culture, both devices enable greater control over the soluble microenvironment by controlling the transport of molecules to and away from the cells. This approach is particularly relevant for cell types such as embryonic stem cells (ESCs) which are especially sensitive to the microenvironment. We have demonstrated for the first time culture of murine ESCs (mESCs) in continuous, logarithmically scaled perfusion for 4 days, with flow rates varying >300× across the array. Cells grown in the slowest flow rate did not proliferate, while colonies grown in higher flow rates exhibited healthy round morphology. We have also demonstrated logarithmically scaled continuous perfusion culture of 3T3 fibroblasts for 3 days, with proliferation at all flow rates except the slowest rate.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...