The open molecular framework of paramagnetic lithium octabutoxy-naphthalocyanine: implications for the detection of oxygen and nitric oxide using EPR spectroscopy†
Abstract
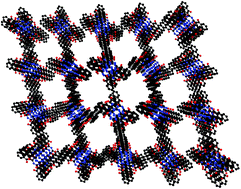
The oxygen-induced broadening of the electron paramagnetic resonance (EPR) spectrum of lithium octa-n-butoxy-naphthalocyanine (LiNc-BuO) is interpreted in terms of its open molecular framework crystal structure. LiNc-BuO was prepared as a microcrystalline powder and its structure analyzed using X-ray powder diffraction techniques. The structure contains strongly coupled dimers of LiNc-BuO molecules, which favors a high degree of spin exchange, and results in a single sharp EPR line. The molecular packing leads to a structure with open channels large enough (10 × 6 Å2) for the penetration of small diatomic paramagnetic molecules such as oxygen (O2) and nitric oxide (NO), as well as the larger triatomic species, nitrogen dioxide (NO2). The EPR linewidth of LiNc-BuO is extremely sensitive to the concentration of paramagnetic gases in the pressure range of 0–760 mmHg. The effect of oxygen on LiNc-BuO is reversible without any signs of permanent adsorption or chemical oxidation. The time response of the effect of oxygen is extremely rapid (0.24 s). The paramagnetic gas-sensing properties of LiNc-BuO are attributed to the open molecular framework of the crystal structure. The oxygen-sensing property, combined with the previously established biostability and biocompatibility of this material, should enable precise and accurate measurements of oxygen concentration in biological systems using EPR spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...