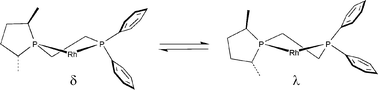
The new unsymmetrical, optically active ligands 1,2-C2H4(PPh2)(2′R,5′R-2′,5′-dimethylphospholanyl) (Laa) and 1,3-C3H6(PPh2)(2′R,5′R-2′,5′-dimethylphospholanyl) (Lbb) form complexes of the type [Rh(L)(cyclooctadiene)][BF4] where L = Laa (1a) or Lbb (1b), [PtCl2(L)] where L = Laa (2a) or Lbb (2b) and [PdCl2(L)] where L = Laa (3a) or Lbb (3b). The crystal structures of 2a and 2b show the chelate ligand backbones adopt δ-twist and flattened chair conformations respectively. Asymmetric hydrogenation of enamides and dehydroaminoesters using 1a and 1b as catalysts show that the ethylene-backboned diphosphine Laa gives a more efficient catalyst in terms of asymmetric induction than the propylene-backboned analogue Lbb. The greatest enantioselectivities were obtained with 1a and enamide substrates with ees up to 91%. Substrate-induced conformational changes in the Rh–diphosphine chelates are proposed to explain some of the ees observed in the hydrogenation of enamides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...