Detection of hydrogen bonding in solution: A 2H nuclear magnetic resonance method based on rotational motion of a donor/acceptor complex†
Abstract
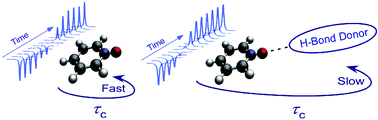
The effect of hydrogen bonding on the rotational correlation time of an H-bond acceptor, pyridine N-oxide-d5, in various

 Please wait while we load your content...
Please wait while we load your content...