The heterogeneous kinetics of HOBr and HOCl on acidified sea salt and model aerosol at 40–90% relative humidity and ambient temperature
Abstract
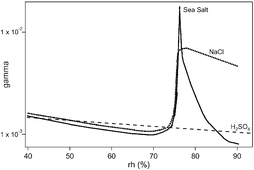
The HOBr and HOCl uptake coefficient γ on H2SO4-acidified submicron salt aerosol of known size distribution was measured in an atmospheric pressure laminar flow reactor. The interaction time of the trace gas with the aerosol was in the range 15 to 90 s and led to γ values in the range 10−4 to 10−2. The acidity of the aerosol is essential in order to enable heterogeneous reactions of HOBr on NaCl, recrystallized sea salt (RSS) and natural sea salt (NSS) aerosols. Specifically, HOCl only reacts on acidified NSS aerosol with a γ ranging from 0.4 × 10−3 to 1.8 × 10−3 at a relative humidity (rh) at 40 and 85%, respectively. Uptake experiments of HOBr on aqueous H2SO4 as well as on H2SO4-acidified NaCl, RSS or NSS aerosol were performed for rh ranging from 40 to 93%. The γ value of HOBr on acidified NSS reaches a maximum γ = 1.9 × 10−2 at rh = 76 ± 1% and significantly decreases with increasing rh in contrast to acidified NaCl and RSS aerosols whose γ values remain high at γ = (1.0 ± 0.2) × 10−2 at rh ≥ 80%. An explanation based on the formation of an organic coating on NSS aerosol with increasing rh is proposed.

 Please wait while we load your content...
Please wait while we load your content...