Structural and mechanistic studies on anthocyanidin synthase catalysed oxidation of flavanone substrates: the effect of C-2 stereochemistry on product selectivity and mechanism
Abstract
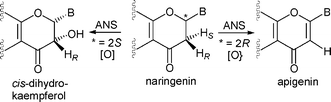
During the biosynthesis of the tricyclic flavonoid natural products in plants, oxidative modifications to the central C-ring are catalysed by Fe(II) and 2-oxoglutarate dependent oxygenases. The reactions catalysed by three of these enzymes; flavone synthase I, flavonol synthase and anthocyanidin synthase (ANS), are formally desaturations. In comparison, flavanone 3β-hydroxylase catalyses hydroxylation at the C-3 pro-R position of 2S-naringenin. Incubation of ANS with the unnatural substrate (±)-naringenin results in predominantly C-3 hydroxylation to give cis-dihydrokaempferol as the major product; trans-dihydrokaempferol and the desaturation product, apigenin are also observed. Labelling studies have demonstrated that some of the formal desaturation reactions catalysed by ANS proceed via initial C-3 hydroxylation followed by dehydration at the active site. We describe analyses of the reaction of ANS with 2S- and 2R-naringenin substrates, including the anaerobic crystal structure of an ANS–Fe–2-oxoglutarate–naringenin complex. Together the results reveal that for the ‘natural’ C-2 stereochemistry of 2S-naringenin, C-3 hydroxylation predominates (>9 : 1) over desaturation, probably due to the inaccessibility of the C-2 hydrogen to the iron centre. For the 2R-naringenin substrate, desaturation is significantly increased relative to C-3 hydroxylation (ca. 1 : 1); this is probably a result of both the C-3 pro-S and C-2 hydrogen atoms being accessible to the reactive oxidising intermediate in this substrate. In contrast to the hydroxylation–elimination desaturation mechanism for some ANS substrates, the results imply that the ANS catalysed desaturation of 2R-naringenin to form apigenin proceeds with a syn-arrangement of eliminated hydrogen atoms and notvia an oxygenated (gem-diol) flavonoid intermediate. Thus, by utilising flavonoid substrates with different C-2 stereochemistries, the balance between C-3 hydroxylation or C-2, C-3 desaturation mechanisms can be altered.

 Please wait while we load your content...
Please wait while we load your content...