Synthesis of achiral and racemic catenanes based on terpyridine and a directionalized terpyridine mimic, pyridyl-phenanthroline
Abstract
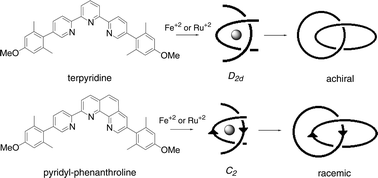
Concatenated macrocycles containing manisyl-substituted tridentate ligands 2,2′:6′,2″-terpyridine and 2-pyridin-2-yl-1,10-phenanthroline (simply referred to as terpyridine and pyridyl-phenanthroline herein) have been prepared via dual cyclization procedures. The manisyl derivative (manisyl = 4-methoxy-2,6-dimethylphenyl) was chosen for its ability to improve solubility while simultaneously incorporating functionality. Deprotection of the methoxy groups provided a soluble ligand that was re-alkylated with an array of terminal alkyne and alkene linkers. The tridentate coordinating ability of these ligands enabled complexation with Ru(II) and Fe(II), generating achiral and racemic octahedral complexes for terpyridine and pyridyl-phenanthroline, respectively. Subsequent macrocyclization via olefin metathesis or copper-mediated alkyne coupling afforded the corresponding catenanes, and in some cases a figure-eight macrocycle. The difference in symmetry and the presence of the manisyl group allowed the distinction between the catenane and the undesired figure-eight to be made directly by 1H NMR. Metal-free achiral and racemic catenanes were obtained by liberating Fe(II) from the octahedral bound title ligands by treatment with hydrogen peroxide.

 Please wait while we load your content...
Please wait while we load your content...