Synthesis of protected γ-carboxyglutamates and γ-acylglutamates by rearrangement of N,N-diacylglutamates†
Abstract
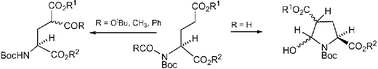
A new method for γ-acylation of protected glutamic acids, involving intramolecular rearrangement of an acyl urethane, has been devised to prepare the protected γ-carboxyglutamates 7, 9 and 11 and the protected 4-acylglutamates 15 and 22 from N,N-bisurethanes or N-acyl-N-urethanes of general structure 1. When the formyl-urethane 17 was used in the reaction, then the intermediate 18 in the intramolecular rearrangement was obtained.

 Please wait while we load your content...
Please wait while we load your content...