Chemoenzymatic synthesis of carbasugars from iodobenzene
Abstract
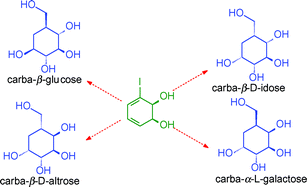
The versatile enantiopure cis-dihydrodiol metabolite 1, formed by bacterial metabolism of iodobenzene, has been used for the synthesis of the pyranose carbasugars (pseudosugars) carba-β-D-altropyranose 2, carba-α-L-galactopyranose 3, carba-β-D-idopyranose 4 and carba-β-L-glucopyranose 5. Substitution of the iodine atom by a carbomethoxy group, stereoselective catalytic hydrogenation of an α,β-unsaturated ester, and regioselective inversion of one or two allylic chiral centres are the key steps used in the synthesis of carbasugars 2–5. The relative and absolute configurations of compounds 2–5 were established by a combination of stereochemical correlation, X-ray crystallography and 1H-NMR spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...