Synthesis of heteroarenes using cascade radical cyclisation via iminyl radicals†
Abstract
Cascade radical cyclisation involving homolytic aromatic substitution has been used to synthesise new tetracycles. Treatment of vinyl iodide radical precursors with Me3Sn˙ radicals (from hexamethylditin) yielded intermediate vinyl radicals which undergo 5-exo cyclisation onto suitably placed nitrile groups to yield intermediate iminyl radicals. The iminyl radicals undergo aromatic homolytic substitution via 6-endo cyclisation (or 5-exo cyclisation followed by neophyl rearrangement) with loss of hydrogen (H˙) in a H-abstraction step. We propose that this abstraction was facilitated by tert-butoxyl (t-BuO˙) radicals from di-tert-butyl peroxide or methyl radicals, generated from breakdown of trimethylstannyl radicals (Me3Sn˙). The biologically active alkaloids mappicine and luotonin A were synthesised using the new methodology. A novel radical conversion of nitriles to primary amides is proposed.
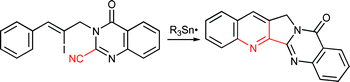

 Please wait while we load your content...
Please wait while we load your content...