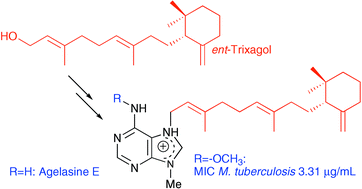
Agelasine E, previously isolated from the marine sponge Agelas nakamurai, has been synthesized for the first time, together with analogs with various terpenoid side chains. Treatment of N6-methoxy-9-methyl-9H-purin-6-amine with allylic bromides gave the desired 7,9-dialkylpurinium salts together with minor amounts of the N6-alkylated isomer. The N6-methoxy group was finally removed reductively. 1H–15N HMBC and 1H–15N HSQC NMR spectroscopy gave additional information on tautomerism and charge delocalization in the purine derivatives studied. The heterocyclic products were screened for activity against Mycobacterium tuberculosis and agelasine analogs carrying a relatively long terpenoid substituent in the purine 7-position and a methoxy group at N-6 were potent inhibitors of bacterial growth. Since agelasine analogs with the geranylgeranyl chain at N-7 exhibited antimicrobial activity, several strategies for synthesis of geometrically pure (2E,6E,10E)-geranylgeranyl bromide from geranyllinalool were evaluated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...