Phospholidines incorporating a β N-sulfonylaminoalcohol moiety: first observed selectivity of phosphorus heterocycle aminolysis in the presence of water†
Abstract
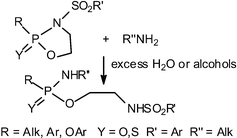
Eleven 2-oxo or 2-thioxo 3-sulfonyl 1,3,2-oxazaphospholidines were synthesized in one step by condensing P(IV) dichlorides with N-sulfonyl-ethanolamines, or -aminothexylalcohols or -ortho-aminophenols. These compounds, in contrast to all other phosphorus heterocycles studied so far, reacted easily with

 Please wait while we load your content...
Please wait while we load your content...