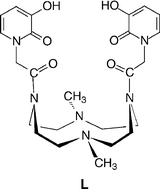
The equilibria and kinetics of the binding of gallium(III) to 4-(N),10-(N)-bis[2-(3-hydroxo-2-oxo-2-H-pyridine-1-y1)acetamido]-1,7-dimethyl-1,4,7,10-tetraazacyclododecane (L) were investigated in acidic medium at ionic strength 1 M (NaClO4). Spectrophotometric titrations in the UV region revealed that L is able to bind Ga3+ also at high H+ concentration. The kinetic (stopped-flow) experiments are interpreted on the basis of three parallel reaction paths (i) M3+
+ H2L2+
= M(H2L)5+ where M(H2L)5+ is in a steady state, (ii) M(OH)2+
+ H2L2+
= M(HL)4+
+ H2O and (iii) M(OH)2+
+ HL+
= ML3+
+ H2O. The first-order rate constants for conversion of the outer-sphere into the inner-sphere complexes are similar to those of the Ga(III)/tropolone system which is known to react according to the dissociative Id mechanism and to the relevant rate constants for water exchange at the metal ion. The effects of pH on the UV-Vis absorption, fluorescence emission properties and NMR spectral features on the Ga(III)/L system were also investigated. Spectrophotometric titrations in the UV region reveal that, in acid medium the prevailing species is M(HL)4+ whereas the chelate ML3+ prevails for [H+] < 0.01 M. The results indicate metal coordination at the oxygen atoms of the 3-hydroxo-2-oxopyridine residues.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...