Stannyl cyclopropanes by diastereoselective cyclopropanations with (tributylstannyl)-diazoacetate esters catalyzed by Cu(i) N-heterocyclic carbene†
Abstract
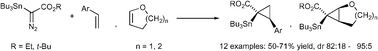
Catalyzed cyclopropanations of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N2)CO2R (R = Et, t-Bu) have been achieved in good yield with excellent diastereoselectivity to make stannyl cyclopropanes having two or three stereocenters, one of which is quaternary.
N2)CO2R (R = Et, t-Bu) have been achieved in good yield with excellent diastereoselectivity to make stannyl cyclopropanes having two or three stereocenters, one of which is quaternary.

 Please wait while we load your content...
Please wait while we load your content...