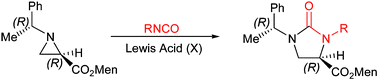
Lewis acid-catalyzed stereospecific ring expansion of aziridine-2-carboxylates to imidazolidin-2-ones†
Abstract
Lewis acid-catalyzed ring expansion reaction of chiral aziridine-2-carboxylate proceeds regio- and stereospecifically to yield enantiomerically pure 4-functionalized imidazolidin-2-ones in high yields.

 Please wait while we load your content...
Please wait while we load your content...