Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction
Abstract
The conversion of 5-aminoimidazole ribonucleotide (AIR) into 4-amino-2-methyl-5-hydroxymethylpyrimidine (HMP) is a fascinating reaction on the thiamin biosynthetic pathway in bacteria and is probably the most complex unresolved rearrangement in primary metabolism. We have successfully reconstituted this reaction in a cell-free system. The E. coli thiC gene product and an additional unidentified E. coli protein are required for the reaction. In addition, SAM and nicotinamide cofactors are required for full activity. Labeling studies to determine the origin of most of the atoms in the pyrimidine are described. Based on these studies, a new mechanism for HMP formation is proposed.
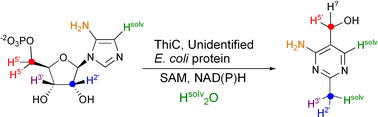

 Please wait while we load your content...
Please wait while we load your content...