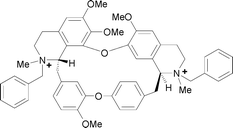
Complexation of free and N-acetylated α-amino acid anions (Gly, Ala, Phe) and some structurally related guests by a dicationic cyclophane-type N,N′-dibenzylated chiral derivative of a bisisoquinoline macrocyclic alkaloid S,S-(+)-tetrandrine (DBT) has been studied by 1H-NMR titrations in D2O. In contrast to other macrocyclic hosts like cyclodextrins and calixarenes, DBT shows highest affinity and large enantioselectivity (K(S)/K(R)
≥ 10) toward smaller N-acetylalanine and binds larger phenylalanine derivatives more weakly and non-selectively. With 1,2,3,4-tetrahydroisoquinoline-3-carboxylate, a rigid analog of phenylalanine, binding again becomes enantioselective with K(S)/K(R)
= 3.8. The binding specificity of DBT is rationalized on the basis of molecular mechanics calculations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...