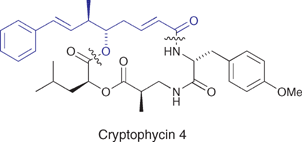
The nucleophilic addition of α-metallated 1,3-dioxanes to planar chiral cationic η3-allylmolybdenum complexes. Synthesis of (2E,5S,6R,7E)-6-methyl-8-phenylocta-2,7-dienoic acid methyl ester, a key component of the Cryptophycins
Abstract
Two adjacent stereogenic centres and a pendant

 Please wait while we load your content...
Please wait while we load your content...