Nucleophilic displacement on 4-nitrophenyl dimethyl phosphinate by ethoxide ion: alkali metal ion catalysis and mechanism
Abstract
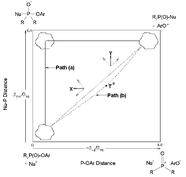
We report on a spectrophotometric kinetic study of the effect of Li+ and K+ cations on the ethanolysis of 4-nitrophenyl dimethylphosphinate (4a) in ethanol at 25 °C. The nucleophilic displacement reaction of 4a with LiOEt and KOEt in the absence and presence of 18-crown-6 ether (18-C-6) furnished observed first-order rate constants which increase in the order EtO− < KOEt < LiOEt. The kinetic data are analyzed in terms of a scheme which assigns concurrent kinetic activity to free ethoxide and metal alkoxide, to obtain the second-order rate coefficients for reaction of the metal ion–ethoxide pairs, kMOEt. Derived δGip, δGts and ΔGcat values quantify ground state and transition state stabilization by the metal ions to give δGts > δGip for Li+ and δGts ∼ δGip for K+. These results indicate moderate catalysis by Li+, with 4a manifesting lesser susceptibility to catalysis than other substrates previously studied. Second-order rate constants for the reaction of the aryl dimethylphosphinates 4a–f with free EtO− were obtained from plots of log kobsvs. [KOEt], measured in the presence of excess 18-C-6. Hammett plots with σ and σ° substituent constants give significantly better correlation of rates than σ− and yield a moderately large ρ(ρ°) value; this is interpreted in terms of a stepwise mechanism involving rate-limiting formation of a pentacoordinate intermediate. Comparison of the present results with those of Williams on the aqueous alkaline hydrolysis of Me2P(O)–OPhX and Ph2P(O)–OPhX esters, establishes the rationale for a change in mechanism in the more basic EtO−/EtOH nucleophile/solvent system by a stepwise mechanism instead of a concerted one in aqueous base. Structure–reactivity correlations following Jencks show that the change in mechanism is accounted for by cross interactions between the nucleophile and the leaving group in the transition state. The observed duality of mechanism is rationalized on the basis of the More O'Ferrall–Jencks diagram, as a spectrum of transition states covering a wide range of nucleophile and leaving group basicities.

 Please wait while we load your content...
Please wait while we load your content...