Hydrolytic reactions of 3′-N-phosphoramidate and 3′-N-thiophosphoramidate analogs of thymidylyl-3′,5′-thymidine
Abstract
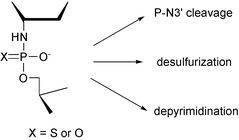
The diastereomeric thiophosphoramidate analogs [(RP)- and (SP)-3′,5′-Tnp(s)T] 2 and the phosphoramidate analog [3′,5′-TnpT] 3 of thymidylyl-3′,5′-thymidine were prepared and their hydrolytic reactions over the pH-range 1–8 at 363.2 K were followed by RP HPLC. At pH < 6, an acid-catalyzed P–N3′ bond cleavage (first-order in [H+]) takes place with both 3′,5′-Tnp(s)T and 3′,5′-TnpT, the former being about 12 fold more stable than the latter. At pH > 4, Tnp(s)T undergoes two competing pH-independent reactions, desulfurization (yielding TnpT) and depyrimidination (cleavage of the N-glycosidic bond) the rates of which are of the same order of magnitude. Also with 3′,5′-TnpT the pH-independent depyrimidination competes with P–N3′ cleavage at pH > 5.

 Please wait while we load your content...
Please wait while we load your content...