A cautionary warning on the use of electrochemical measurements to calculate comproportionation constants for mixed-valence compounds†
Abstract
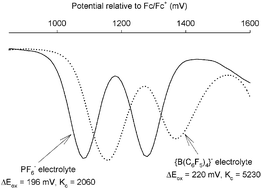
The redox potentials for successive oxidations in a series of ligand-bridged dinuclear ruthenium complexes are shown to be dependent on the identity of the anion used as the electrolyte in the electrochemical measurements. Since the differences between these redox potentials (ΔEox) are often used to calculate the comproportionation equilibrium constants (Kc) in mixed-valence species—and therefore the extent of inter-metal communication between the metal centres—the results demonstrate the need for extreme care in comparison of ΔEox data and of Kc values derived from them.

 Please wait while we load your content...
Please wait while we load your content...