Solid-state spectroscopic properties and the geometry of binuclear rhodium(i) diisocyanoalkane complexes†
Abstract
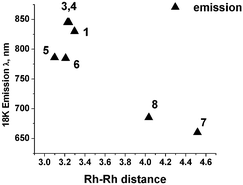
A series of crystalline dinuclear rhodium complexes with different bridging diisocyano ligands and different counter ions have been studied by low-temperature crystallographic and solid-state spectroscopic techniques. The Rh–Rh distances vary from 4.5153(3) to 3.0988(7) Å, and the twist angles around the Rh–Rh line from 58.3(1) to 0°, both depending on the size and conformational rigidity of the bridging ligand. For very long distances as occur in the [Rh2(dimen)4]2+ salts the absorption is significantly blue-shifted compared to other complexes. For a given cation a shorter Rh–Rh bond gives a red shift of the phosphorescence emission band, indicating a smaller energy gap between the ground and emitting excited states. An exception occurs for the [Rh2(1,6-diisocyanohexane)4]2+ ion, in which dimer formation in the calixarate salt lengthens the Rh–Rh intramolecular bond length without affecting the emission spectrum.

 Please wait while we load your content...
Please wait while we load your content...