Remarkable effect of 2α-modification on the VDR antagonistic activity of 1α-hydroxyvitamin D3-26,23-lactones
Abstract
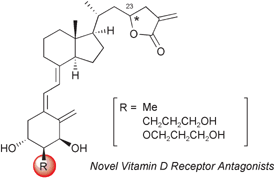
Novel 2α-methyl-, 2α-(3-hydroxypropyl)- and 2α-(3-hydroxypropoxy)-substituted 25-dehydro-1α-hydroxyvitamin D3-26,23-lactone derivatives were efficiently synthesized via Reformatsky type allylation and

 Please wait while we load your content...
Please wait while we load your content...