Does the DABCO-catalysed reaction of 2-hydroxybenzaldehydes with methyl acrylate follow a Baylis–Hillman pathway?
Abstract
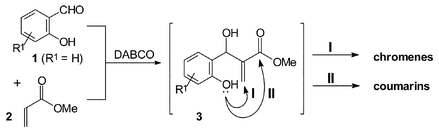
Evidence is presented which supports the intermediacy of dipolar Baylis–Hillman-type adducts in the synthesis of coumarin and chromene derivatives from the reaction of 2-hydroxybenzaldehydes with methyl acrylate in the presence of 1,4-diazabicyclo[2.2.2]octane (DABCO).

 Please wait while we load your content...
Please wait while we load your content...