Enantioselective alkylative double ring-opening of epoxides derived from cyclic allylic ethers: synthesis of enantioenriched unsaturated diols†
Abstract
A screen of external chiral ligands has led to enantioselective organolithium-induced alkylative double ring-opening of 3,4-epoxytetrahydrofuran 1 with n-BuLi to give 3-methyleneheptane-1,2-diol 3 in 75% yield and 55% ee in the presence of bisoxazoline 10, and in up to 60% ee in the presence of (−)-sparteine 2. Extending the alkylative double ring-opening reaction to epoxides derived from oxabicyclo[n.2.1]alkenes (n = 2, 3) results in the formation of cycloalkenediols, which, when carried out in the presence of (−)-sparteine 2 affords products in up to 85% ee.
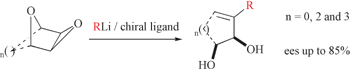

 Please wait while we load your content...
Please wait while we load your content...