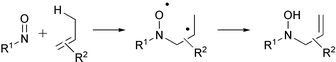
A theoretical study of the mechanisms of ene reactions of nitroso compounds has been completed, using UB3LYP, CASPT2, UCCSD(T) and UQCISD(T) methods. Stepwise paths through polarized diradical intermediates are always preferred. These intermediates have unusual properties, involving high rotational barriers about formally single bonds, which permit them to maintain stereochemical relationships. The diradicals may exchange the RNO moiety between the two ends of the alkene via an aziridine N-oxide. The aziridine N-oxide cannot be accessed directly from reactants and cannot lead directly to ene products. It is therefore an innocent by-stander in the way proposed by Singleton for the aziridinium imide in the ene reactions of triazolinediones. A detailed analysis of the electronic structure of the polarized diradicals is given. The kinetic isotope effects measured in a Stephenson isotope effect test have been reproduced. These kinetic isotope effects are consistent with a mechanism in which partitioning of the polarized diradical between cyclization to an aziridine N-oxide and H-abstraction to ene product takes place, and in which the formation of the polarized diradical is to some extent reversible. Finally, calculated regioselectivities reproduce those observed experimentally.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...