Dehydration of fructose to 5-hydroxymethylfurfural in sub- and supercritical acetone
Abstract
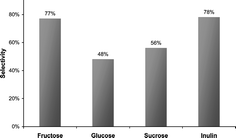
The dehydration of D-fructose (1) to 5-hydroxymethylfurfural (HMF, 2) is a well known reaction (acid-induced elimination of three moles of water), performed by many investigators during the last three decades. HMF and its oxidation product 2,5-furandicarboxylic acid are so called ‘sleeping giants’ in the field of intermediate chemicals from regrowing resources. HMF is a key substance between carbohydrate chemistry and mineral oil-based industrial chemistry and it has the potential of a commodity like terephthalic acid. Surprisingly, no technical process has been constructed until now. The reason is that high selectivities (in this context selectivity means yield divided by conversion in mol%) can only be obtained in high boiling polar solvents like dimethyl sulfoxide, dimethylformamide, acetonitrile, poly(glycol ether) etc. so that separation procedures are very expensive. Unfortunately, in aqueous systems (supercritical water), only low selectivities can be achieved. Considering the specifications for a modern sustainable and economical process, the use of green solvents like acetone, methanol or acetic acid in their sub- or supercritical states should be accounted for. The results for this reaction performed in sub- and supercritical acetone are presented here.

 Please wait while we load your content...
Please wait while we load your content...