New chemistry needed to facilitate the replacement of axial ligands (X) in Ni3(dpa)4X2 complexes (dpa = the anion of dipyridylamine) has been explored and used to replace X = Cl by X = CN, NCNCN, and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh. The resulting compounds, 3, 4, and 5, respectively, have been characterized by X-ray crystallography and cyclic voltammetry, inter alia. It is found that both the mean Ni⋯Ni separations (D), and the magnitude of the antiferromagnetic coupling (J) between the terminal, high spin (S
= 1) Ni(II) atoms vary in a correlated way, with |J| decreasing with increasing D. The relationship is nearly linear over the available ranges of parameters, suggesting that the coupling may proceed mainly through the central, diamagnetic Ni(II) ion.
CPh. The resulting compounds, 3, 4, and 5, respectively, have been characterized by X-ray crystallography and cyclic voltammetry, inter alia. It is found that both the mean Ni⋯Ni separations (D), and the magnitude of the antiferromagnetic coupling (J) between the terminal, high spin (S
= 1) Ni(II) atoms vary in a correlated way, with |J| decreasing with increasing D. The relationship is nearly linear over the available ranges of parameters, suggesting that the coupling may proceed mainly through the central, diamagnetic Ni(II) ion.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh. The resulting compounds, 3, 4, and 5, respectively, have been characterized by
CPh. The resulting compounds, 3, 4, and 5, respectively, have been characterized by 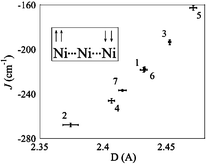

 Please wait while we load your content...
Please wait while we load your content...