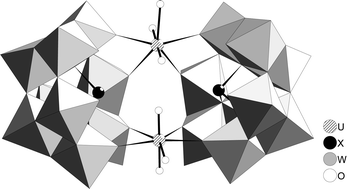
The reaction of UO22+ with the trivacant lacunary polyoxometalate anions, [SbW9O33]9− and [TeW9O33]8−, yields the novel isostructural complexes [(UO2)2(H2O)2(SbW9O33)2]14−
(1) and [(UO2)2(H2O)2(TeW9O33)2]12−
(2), respectively. The complex anions contain two [XW9O33]n−
(X = SbIII or TeIV) anions linked by two UO22+ cations. Each uranyl moiety bonds to two unsaturated oxygen atoms of each lacunary anion in the complex. Each [XW9O33]n− anion has six unsaturated oxygen atoms meaning that in 1 and 2 each [XW9O33]n− anion has two unsaturated oxygen atoms which remain uncoordinated to uranium with the result being the formation of an ‘open’ sandwich structure. The fact that a third UO22+ cation is not coordinated to form a ‘closed’ sandwich structure (as is observed for first row d-block transition metals) is attributed to the steric hindrance of the axial ‘yl’ oxygen atoms of the uranyl group. The products, prepared as NH4+ salts, have been characterised by single crystal X-ray diffraction, elemental analysis, TGA analysis, IR, Raman and UV/vis spectroscopy, which indicate that the O donor atoms of the lacunary heteropolytungstate anions are strongly coordinating to U(VI) in the equatorial plane, weakening the uranyl U–O axial bonds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...