Chemical consequences of fluorine substitution. Part 4.† Diels–Alder reactions of fluorinated p-benzoquinones with Dane's diene. Synthesis of fluorinated D-homosteroids
Abstract
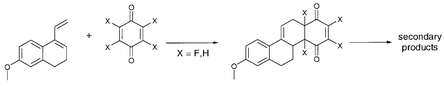
Four fluorinated p-benzoquinones (2) have been reacted with Dane's diene (1) in Diels–Alder reactions and the formed fluorinated D-homosteroids were characterized. The number of products, their stereochemistry and stability depends on the fluorine substitution pattern of the corresponding fluorinated p-benzoquinones. If the p-benzoquinone (2) contains an unfluorinated double bond, this bond reacts faster with diene 1 yielding endo-products selectively. In contrast, [4+2]cycloadditions with 2,6-difluoro (2c) and

 Please wait while we load your content...
Please wait while we load your content...