Synthesis of the first amine–cyanocarboxyboranes, isoelectronic analogues of α-cyanocarboxylic acids
Abstract
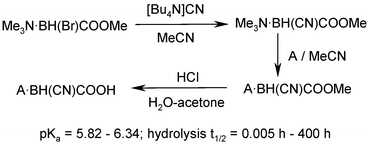
The first amine–cyanocarboxyboranes and their esters are synthesized from trimethylamine–bromo(methoxycarbonyl)boranes with tetrabutylammonium cyanide, followed by amine exchange reactions and acidic hydrolysis; pKa values of “boracyanocarboxylic acids” are determined and their stability in acidic medium examined.

 Please wait while we load your content...
Please wait while we load your content...