Abstract
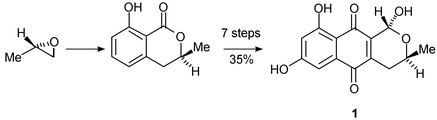
The (1R,3S)-absolute stereochemistry of thysanone 1, a fungal benzoisochromanquinone with potent human rhinovirus 3C-protease inhibitory activity, is established for the first time by total synthesis of the natural product from ethyl (S)-lactate and CD comparison with authentic material.

 Please wait while we load your content...
Please wait while we load your content...