Enantioselective total synthesis of vicenistatin, a novel 20-membered macrocyclic lactam antitumor antibiotic
Abstract
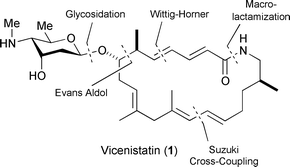
Enantioselective total synthesis of an antitumor antibiotic, vicenistatin, featuring a 20-membered macrocyclic lactam glycoside with the amino sugar vicenisamine, has been achieved. Key reactions in the synthesis of macrolactam aglycone involved Suzuki cross-coupling and Evans asymmetric aldol reaction. Penultimate glycosidation of the O-TMS-aglycone with appropriately protected 1-O-acetyl amino sugar and final deprotection allowed accomplishment of the total synthesis.

 Please wait while we load your content...
Please wait while we load your content...