Synthesis of 2,16α- and 4,16α-difluoroestradiols and their 11β-methoxy derivatives as potential estrogen receptor-binding radiopharmaceuticals
Abstract
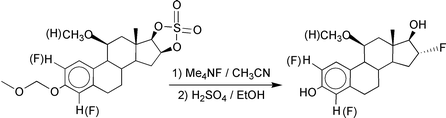
We prepared the 2,16α- and 4,16α-difluoroestradiols and their 11β-methoxy derivatives via two different pathways. The first route permits large scale synthesis and characterization of the final products while the second route was selected to allow for

 Please wait while we load your content...
Please wait while we load your content...