The Baylis–Hillman condensation of α,β-conjugate cycloketones with aldehydes using diethylaluminum iodide alone as the promoter†
Abstract
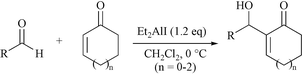
The Baylis-Hillman condensation of three types of α,β-conjugate cycloketones with aldehydes was successfully performed by using diethylaluminum iodide as the Lewis acid promoter alone without the direct use of a Lewis base. The reaction proceeded to completion at 0 °C in CH2Cl2 within 24 h to give modest to good yields (53–72%).

 Please wait while we load your content...
Please wait while we load your content...