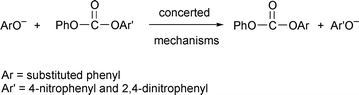
The reactions of phenyl 4-nitrophenyl carbonate (PNPC) and phenyl 2,4-dinitrophenyl carbonate (PDNPC) with a homogeneous series of phenoxide anions are subjected to a kinetic investigation in water at 25.0 °C, ionic strength 0.2 mol dm−3 (KCl). Under phenoxide or total phenol excess over the substrate all these reactions obey pseudo first-order kinetics and are first order in phenoxide. The Brønsted-type plots for the nucleophilic rate constants are linear with slopes 0.61 and 0.49 for the phenolysis of PNPC and PDNPC, respectively. The magnitude of these slopes and the absence of curvature in the Brønsted plot at pKa
= 7.1 for the PNPC reactions are consistent with concerted mechanisms (one step) for both reaction series. PDNPC is more reactive than PNPC toward phenoxide nucleophiles; this can be explained by the presence of a second nitro group in PDNPC, which (i) leaves its carbonyl carbon more positively charged than that of PNPC, making the former a better electrophile, and (ii) makes 2,4-dinitrophenoxide a better leaving group than 4-nitrophenoxide. The larger nucleophilic rate coefficients found in this work relative to those obtained in the concerted phenolysis of 4-nitrophenyl and 2,4-dinitrophenyl methyl carbonates is explained by a stronger electron withdrawal from PhO compared to MeO. Comparison of the concerted phenolysis of PNPC with the stepwise reactions of quinuclidines with the same substrate indicates that substitution of a quinuclidino group in a zwitterionic tetrahedral intermediate by a phenoxy group greatly destabilises the intermediate.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...