Iminoxyl radicals and stable products from the one-electron oxidation of 1-methylindole-3-carbaldehydeoximes
Abstract
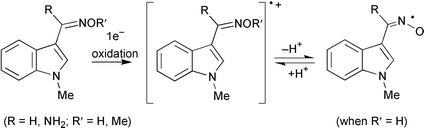
The radical intermediates and the stable products formed on one-electron ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH to yield the radical
NOH to yield the radical ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH]˙+, which exist in prototropic equilibria with the neutral iminoxyl radicals [
NOH]˙+, which exist in prototropic equilibria with the neutral iminoxyl radicals [![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NO]˙ (pKa
= 3.53 ± 0.03 and 5.01 ± 0.01 at ionic strength 0.05 M, respectively). This was confirmed by the observed primary salt-effect which accelerated the decay of the radical
NO]˙ (pKa
= 3.53 ± 0.03 and 5.01 ± 0.01 at ionic strength 0.05 M, respectively). This was confirmed by the observed primary salt-effect which accelerated the decay of the radical ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOCH3]˙+. At low concentrations of 2a and high dose rates the 2a radicals [
NOCH3]˙+. At low concentrations of 2a and high dose rates the 2a radicals [![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NO]˙ decayed bimolecularly via unstable dimers to the
NO]˙ decayed bimolecularly via unstable dimers to the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, with higher concentrations and lower dose rates favouring the chain-catalysed isomerisation of the N-hydroxyimino moiety. Radicals from 4a decay bimolecularly to form unstable dimers which degrade to produce an
O, with higher concentrations and lower dose rates favouring the chain-catalysed isomerisation of the N-hydroxyimino moiety. Radicals from 4a decay bimolecularly to form unstable dimers which degrade to produce an

 Please wait while we load your content...
Please wait while we load your content...