Hydrolytic stability of nucleoside phosphotriesters derived from bis(hydroxymethyl)-1,3-dicarbonyl compounds and their congeners: towards a novel pro-drug strategy for antisense oligonucleotides
Abstract
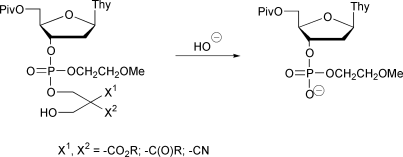
Four different nucleoside phosphodiester protecting groups derived from bis(hydroxymethyl)-1,3-dicarbonyl compounds and their congeners have been prepared and introduced to 5′-O-pivaloylthymidine 3′-(2-methoxyethyl)phosphate and its monothioate analogs. Nucleoside phosphotriesters having either 2,2-bis(ethoxycarbonyl)-3-(4,4′-dimethoxytrityloxy)propyl (1a), 2-cyano-2-methoxycarbonyl-3-(4,4′-dimethoxytrityloxy)propyl (1b), 2,2-bis(cyano)-3-(4,4′-dimethoxytrityloxy)propyl (1c) or 2-acetyl-2-benzoyl-3-(4,4′-dimethoxytrityloxy)propyl (1d) protecting group have been prepared. Additionally were synthesized the O- and S-esterified phosphoromonothioate analogs of 1b. After removal of the dimethoxytrityl group under acidic conditions, each of the detritylated protecting groups is readily cleaved from the phosphate/thiophosphate moiety by a reaction suggested to involve a base-catalyzed retro-aldol condensation and following elimination of the phosphodiester from the formed carbanion intermediate. The kinetics of the hydroxide ion-catalyzed cleavage have been studied by HPLC over a pH range 2–7. The half-lives of the cleavage at pH 7 and 25 °C vary from 0.3 s (for 1c) to 5500 s (for 1a). The results confirm that these protecting groups have promising chemical properties for use in the development of antisense oligonucleotide pro-drug strategies.

 Please wait while we load your content...
Please wait while we load your content...