Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation†
Abstract
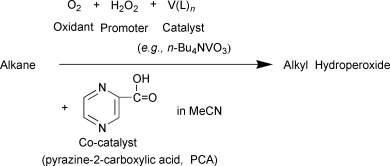
Various combinations of vanadium derivatives (n-Bu4NVO3 is the best catalyst) with pyrazine-2-carboxylic acid (PCA) catalyse the oxidation of saturated hydrocarbons, RH, with hydrogen peroxide and air in acetonitrile solution to produce, at temperatures <40 °C, alkyl hydroperoxides, ROOH, as the main primary products. These compounds are easily reduced with triphenylphosphine to the corresponding alcohols, which can then be quantitatively determined by GLC. Certain aminoacids similar to PCA can play the role of co-catalyst; however the oxidation rates and final product yields are lower for picolinic and imidazole-4,5-dicarboxylic acids, while imidazole-4-carboxylic and pyrazole-3,5-dicarboxylic acids are almost inactive. The oxidation is induced by the attack of a hydroxyl radical on the alkane, RH, to produce alkyl radicals, R˙. The latter further react rapidly with molecular atmospheric oxygen. The peroxyl radicals, ROO˙, thus formed can be converted to alkyl hydroperoxides. We conclude on the basis of our kinetic investigation of the oxidation of cyclohexane that the rate-limiting step of the reaction is the monomolecular decomposition of the complex containing one coordinated PCA molecule: VV(PCA)(H2O2) → VIV(PCA) + HOO˙ + H+. The VIV species thus formed reacts further with a second H2O2 molecule to generate the hydroxyl radical according to the equation VIV(PCA) + H2O2 → VV(PCA) + HO˙ + HO−. The concentration of the active species in the course of the catalytic process has been estimated to be as low as [V(PCA)H2O2] ≊ 3.3 × 10−6 mol dm−3. The effective rate constant for the cyclohexane oxidation (d[ROOH]/dt = keff[H2O2]0[V]0) is keff = 0.44 dm3 mol−1 s−1 at 40 °C, the effective activation energy is 17 ± 2 kcal mol−1. It is assumed that the accelerating role of PCA is due to its facilitating the proton transfer between the oxo and hydroxy ligands of the vanadium complex on the one hand and molecules of hydrogen peroxide and water on the other hand. For example: (pca)(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )V⋯H2O2 → (pca)(HO–)V–OOH. Such a “robot’s arm mechanism” has analogies in enzyme catalysis.
)V⋯H2O2 → (pca)(HO–)V–OOH. Such a “robot’s arm mechanism” has analogies in enzyme catalysis.

 Please wait while we load your content...
Please wait while we load your content...