Short-chain analogues of the lipopeptaibol antibiotic trichogin GA IV: conformational analysis and membrane modifying properties
Abstract
To examine the role of the peptide main-chain length on the conformation and membrane activity of the lipopeptaibol
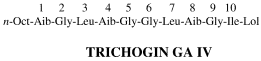

 Please wait while we load your content...
Please wait while we load your content...