Short synthesis of the optically active E-ring portion of (S)-camptothecin
Abstract
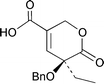
(5S)-5-Benzyloxy-5-ethyl-6-oxo-5,6-dihydro-2H-pyran-3-carboxylic acid, the protected E-ring moiety of (S)-camptothecin, has been rapidly prepared in enantiomerically enriched form (98% ee) through enolate conjugate addition to a β-bromo methacrylate derivative, followed by enzymatic resolution with PLE.

 Please wait while we load your content...
Please wait while we load your content...