Vilsmeier–Haack reaction of tertiary alcohols: formation of functionalised pyridines and naphthyridines
Abstract
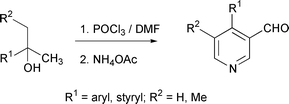
Vilsmeier–Haack reaction of 2-arylpropan-2-ols proceeds with multiple iminoalkylations leading to the formation of conjugated iminium salts which on ammonium acetate-induced cyclisation afford 4-arylnicotinaldehydes in good yields. Tertiary alcohols derived from aliphatic or alicyclic ketones by the addition of methyl Grignard are converted into substituted pyridines and naphthyridines by the action of Vilsmeier's reagent in N,N-dimethylformamide followed by nucleophile-assisted cyclisation in the presence of ammonium acetate.

 Please wait while we load your content...
Please wait while we load your content...