Simple and selective one-pot replacement of the N-methyl group of tertiary amines by quaternization and demethylation with sodium sulfide or potassium thioacetate: an application to the synthesis of pergolide
Abstract
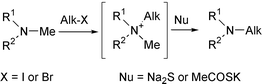
The paper describes a mild, selective, and rapid replacement of an N-methyl group of

 Please wait while we load your content...
Please wait while we load your content...