Intramolecular condensation of 2-(acetonyl)-3-acyljuglone derivatives under basic conditions gave 1,6- and/or 1,8-dihydroxyanthraquinones depending on the conditions employed. Treatment of 6-[(3-acetyl-5-methoxy-1,4-dioxo-1,4-dihydro-2-naphthyl)methyl]-2,2-dimethyl-4H-1,3-dioxin-4-one with K2CO3 in alcohol brought about the intramolecular Knoevenagel-type reaction to give 3-hydroxy-8-methoxy-1-methyl-9,10-dioxo-9,10-dihydro-anthracene-2-carboxylates in good yields, while the same naphthoquinone gave 4-hydroxy-5-methoxy-9,10-dioxo-9,10-dihydroanthracene-2-acetic acid in good yield by treatment with potassium bis(trimethylsilyl)amide (KHMDS).
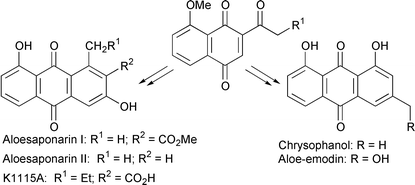
Chrysophanol, aloe-emodin, aloesaponarin I, and K1115A were prepared in good yields.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...